Exploring Emerging Technologies in Hydrogen Energy Production

Introduction
As the world seeks sustainable and clean energy solutions, hydrogen has emerged as a promising contender, and a promising alternative to the conventional fossil fuels. With its high energy density and ability to produce electricity without carbon emissions, hydrogen has gained attention as a key player in the transition to a greener future and low-carbon economy. In this blog, we will dive into the exciting realm of emerging technologies in hydrogen energy production, highlighting their potential and impact on our energy landscape.highlighting the advantages, feasibility, and potential for implementation in the Nigerian context.
The Rise of Hydrogen Energy:
Before we explore the cutting-edge technologies, let’s first understand why hydrogen is garnering so much interest. According to the International Renewable Energy Agency’s (IRENA) 2021 World Energy Transitions Outlook, hydrogen and its derivatives will account for 12% of final energy consumption by 2050. Its versatility as a fuel, ability to store renewable energy, and zero-emission properties make it a compelling solution for decarbonizing various sectors, including transportation, industry, and power generation. Additionally, as global awareness of climate change intensifies, the demand for clean energy solutions such as hydrogen continues to grow and gain traction.
Green Hydrogen: The Future Unleashed
One of the most exciting developments in hydrogen energy production is the emergence of green hydrogen. Unlike gray hydrogen, which is produced from fossil fuels, green hydrogen is generated through electrolysis, using renewable energy sources such as solar or wind power. This technology enables the production of hydrogen without any carbon emissions, making it a truly sustainable solution. Also, with the decreasing costs of renewable energy, green hydrogen is fast becoming an economically viable and environmentally friendly option
Advanced Electrolysis Technologies
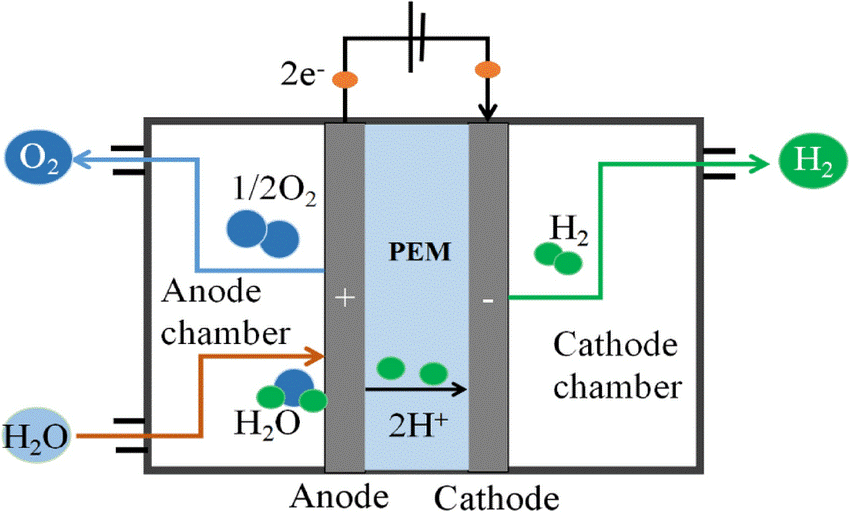
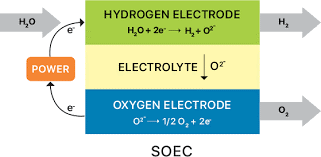
A crucial component of green hydrogen production is electrolysis. Recent advancements in electrolysis technologies have made the process more efficient, cost-effective, and scalable. Proton exchange membrane (PEM) electrolysis and solid oxide electrolysis cells (SOECs) are two types of electrolysis technologies that can provide high-energy conversion efficiencies for H2O and CO2 electrolysis to sustainably produce hydrogen and low carbon fuels.
A research article on Nature Energy explains Proton exchange membrane (PEM) electrolysis as the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. In PEM electrolysis, an electric current is passed through water, causing it to split into hydrogen and oxygen. The hydrogen ions (protons) produced at the anode are conducted through the membrane to the cathode, where they combine with electrons to form hydrogen gas.
Solid oxide electrolysis cells (SOECs) on the other hand, are a type of high-temperature electrolysis cell that can be used for the production of hydrogen from steam or syngas from carbon dioxide and steam based on an article ScienceDirect. SOECs operate at high temperatures (typically 700-800°C) and use a solid oxide as the electrolyte. At these high temperatures, steam can be split into hydrogen and oxygen at the cell’s two electrodes. The oxygen ions produced at the anode are conducted through the solid oxide electrolyte to the cathode, where they combine with electrons to form oxygen gas.
These two technologies are gaining momentum due to their ability to operate at higher temperatures, reducing energy requirements and increasing overall efficiency.
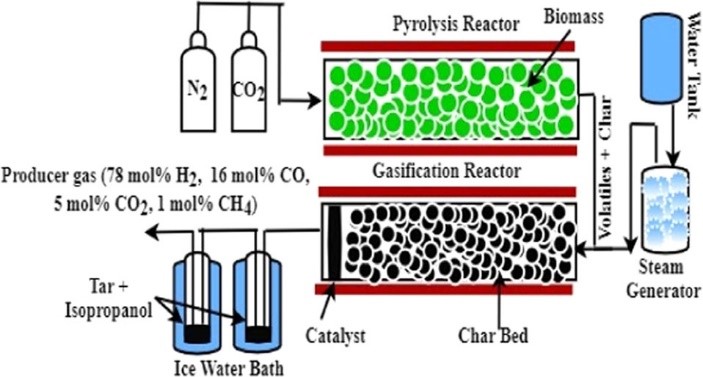
Biomass Gasification: An Innovative Approach
Another promising technology for hydrogen production is biomass gasification. This process involves converting organic materials, such as agricultural waste or wood chips, into a hydrogen-rich gas through partial oxidation. Biomass gasification not only offers a renewable feedstock but also provides a solution for waste management and offers carbon-neutral pathway for hydrogen production as well as the reduction of greenhouse gas emissions.
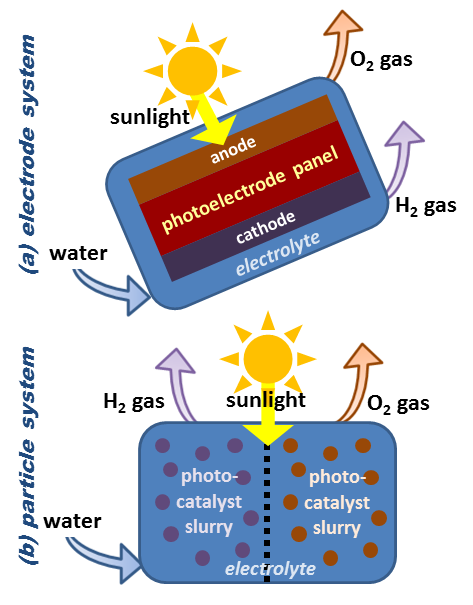
Photobiological and Photoelectrochemical Water Splitting
Nature has inspired innovative approaches to hydrogen production, including photobiological and photoelectrochemical water splitting. For instance, The Office of Energy Efficiency and Renewable Energy explains in details the photoelectrochemical (PEC) water splitting process where hydrogen is produced from water using sunlight and specialized semiconductors called photoelectrochemical materials, which use light energy to directly dissociate water molecules into hydrogen and oxygen. The PEC water splitting process uses semiconductor materials to convert solar energy directly to chemical energy in the form of hydrogen. The semiconductor materials used in the PEC process are similar to those used in photovoltaic solar electricity generation, but for PEC applications the semiconductor is immersed in a water-based electrolyte, where sunlight energizes the water-splitting process Overall, these methods use sunlight to trigger chemical reactions that split water molecules, releasing hydrogen. Through genetically modified microorganisms or specialized semiconductor materials, scientists are harnessing the power of light to generate hydrogen in a sustainable and efficient manner which also enhances the scalability of hydrogen production.
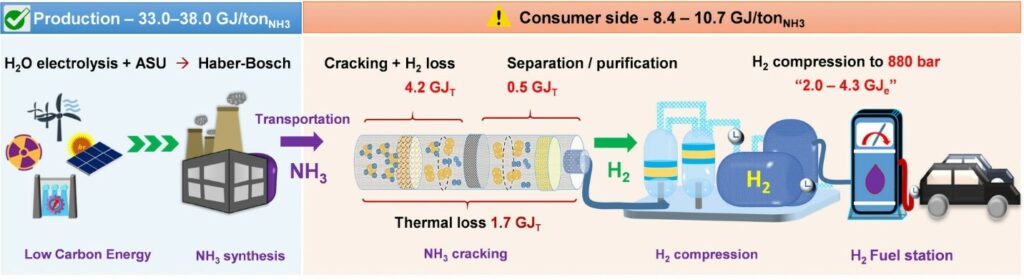
Hydrogen from Renewable Ammonia:
Ammonia, primarily used as a fertilizer, can also be a carrier for hydrogen. By extracting hydrogen from ammonia, a carbon-free fuel source is obtained. The idea of using ammonia as a carrier for hydrogen delivery has gained traction in recent years. This is because ammonia is much easier to liquify than hydrogen and is therefore much easier to store and transport based on research by Northwestern University. The process was further explained as one that functions at much lower temperatures than traditional methods (250 degrees Celsius as opposed to 500 to 600 degrees Celsius) and generates pure hydrogen that does not need to be separated from any unreacted ammonia or other products. The process is efficient because all of the electrical current supplied to the device directly produces hydrogen, without any loss to parasitic reactions. This approach provides an additional pathway for storing and transporting hydrogen, especially for regions with limited infrastructure.
Conclusion
The future of energy is intertwined with hydrogen, and emerging technologies are unlocking its full potential. From advanced electrolysis to biomass gasification and photobiological water splitting, these innovative approaches offer sustainable solutions to meet our energy needs. With ongoing research, collaboration, and investments, hydrogen energy production is set to reshape our energy landscape, leading us towards a cleaner and more sustainable future.
Remember, the power of hydrogen lies not only in its energy capacity but also in its ability to combat climate change and create a greener planet. Let us embrace these emerging technologies and propel the hydrogen revolution forward.
https://www.iea.org/reports/the-future-of-hydrogen
https://www.researchgate.net/journal/Clean-Technologies-and-Environmental-Policy-1618-9558
https://pubs.acs.org/doi/10.1021/acs.iecr.1c01810
https://www.energy.gov/eere/fuelcells/hydrogen-production-photoelectrochemical-water-splitting
https://pubs.acs.org/doi/10.1021/acsenergylett.1c02189





1 Comment
[…] Exploring Emerging Technologies in Hydrogen Energy Production […]