
What is Hydrogen Energy?
Hydrogen, a pure gas, results in the production of water vapour when burnt. Hydrogen is extremely flexible because it can be used as a gas or a liquid and transformed into electricity or fuel.
According to statistics, around 70 million metric tons of hydrogen are produced annually worldwide for use in processes including oil refining, steel production, chemical and fertilizer production, ammonia generation, food processing, and metallurgy.

Types of Hydrogen Energy
Different colours, such as brown, grey, blue, or green, are associated with hydrogen production. The differences between these descriptions are directly related to the quantity of carbon emitted into the atmosphere in the process of producing hydrogen.
Brown Hydrogen
Made → Coal (Gasification) CO2 is emitted into the environment (Most environmentally damaging).
Grey Hydrogen
Made → Natural Gas (SMR) CO2 is emitted into the environment (The most common form of hydrogen).

100% Renewable Energy
Hydrogen is the most abundant of all elements in the universe which exists in different forms. As an energy carrier, it can be produced from various sources of energy such as natural gas, and renewable energy sources.

Zero Carbon Emission
Compared with the use of fossil fuels and nuclear energy, hydrogen is safer and can be produced and consumed through processes with zero impact on the environment.

High-Density Energy Source
Hydrogen has very high energy density by mass and very low energy density by volume which can be stored for use as fuel in internal combustion engines (ICE) and fuel cell vehicles (FEV).
A use case for Hydrogen in Nigeria

Hydrogen Production
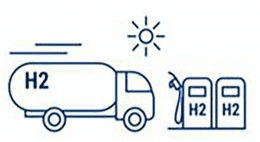
Hydrogen Storage & Distribution
Hydrogen Utilization
Hydrogen production technologies
Steam Methane Reformation (SMR)
- SMR is the process of producing hydrogen from natural gas and water in steam form. Carbon dioxide is released into the environment through this process and the hydrogen produced is called grey hydrogen.
- However, if the carbon dioxide is captured and stored, the hydrogen produced is known as blue hydrogen.
- SMR is used to produce most of the hydrogen available worldwide. Some of the energy content of the produced hydrogen is usually lost as excess heat during the process.
Methane Pyrolysis
- Methane pyrolysis is a one-step method of producing hydrogen from natural gas in high volume at low cost. In this process, methane is directly converted to hydrogen fuel and separable solid carbon through a molten metal catalyst at a high temperature (1065oC).
- The solid carbon is removed and sold as a manufacturing feedstock or landfilled without emission into the environment.
- The hydrogen produced through this process is called turquoise hydrogen.
Electrolysis
- Hydrogen is produced through electrolysis by using electricity renewable energy sources such as solar and wind to split water into hydrogen and oxygen.
- No carbon is emitted through this process and the hydrogen produced is called green hydrogen.
- Only 2 % of hydrogen is produced globally through electrolysis.

